Here we will look at the best manner in which chemistry can be revised.
Every question must be read critically and given the correct answers as wrong answers invite negative marking.
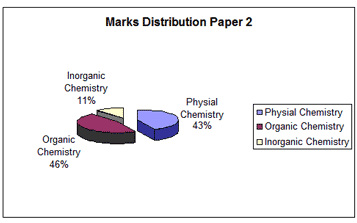
SI units are followed in physical chemistry. Proper conversion to uniform units for terms for example like pressure, volume, temperature while solving numerical problems would go a long way in arriving at the correct results.
Questions on topics like atomic structure and chemical bonding find a place every year. It is absolutely necessary to prepare a table containing essential data to be used as a ready reckoner and a quick glance. This table must contain information like shortened form of equations for ready substitution, mathematical forms of definitions in topics like gas laws, kinetics, thermodynamics, chemical kinetics and ionic equilibria and other related titles in physical chemistry, for ready reference.
Special attention has to be focused on electrochemistry particularly relating to cell reactions, EMF, Oxidation-reduction half cells and standard potentials. Although the entire question paper is of the objective type, questions requiring a few steps of calculation in physical chemistry topics.
Coordination compounds and extractive metallurgy are the two major areas in inorganic chemistry. Emphasis is laid on the hybridization and geometries and magnetic properties of complexes. Extraction principles like self reduction, electrolytic reduction, carbon reduction involving the elements Cu, Pb, Mg, Al, Fe and Sn are very important.
Extraction of Ag and Au by cyanide process is another topic form which questions are often asked. Oxides and oxyacids of N, P, halogens, S are important from the point of view of oxidation states of elements, strength of oxyacids and their oxidising properties.
Application of principles of solubility product in inorganic qualitative analysis is important. Structures of a few inorganic compounds like AlCl3, B2H6, H2O2, silicates can be asked.
Organic chemistry forms the third major part of study material. Though the structures, mechanisms and reactions are a bit hard to remember, this is a branch of chemistry where there is scope for scoring full marks.
Basic organic chemistry includes nomenclature particularly the IUPAC system, and the electronic effects. Sufficient attention has to be focused on these topics as these principles constitute the ground work for higher learning of organic chemistry.
Knowledge of important name reactions, molecular rearrangements with some mechanistic details relating to these topics would be of immense help in clearing questions in organic chemistry. Products of commercial importance like polymers, naturally occurring materials like carbohydrates, proteins are also important. Stereochemistry is a topic that should not be ignored.
The pattern of the IIT JEE paper has seen a major change in the last few years. The same subject with full coverage of the syllabus has to be answered in both the sessions of the examination on the same day.
One hour available for each subject in the morning and the evening sessions should be carefully divided in answering all the questions. Multiple choice questions need careful study prior to answering as only one answer is absolutely correct and it is possible that the remaining three options are also partially correct.
Passages for comprehension-based questions are generally not adequate to give all the information needed to answer the questions that follow. Matrix matching type questions need particular attention as either you score full marks or you end up with a naught.
Wishing you the very best in securing the high ranks in the ensuing exam on April 13.
0 comments:
Post a Comment